Bevacizumab (Bev) in combination with XELOX or FOLFOX-4: updated efficacy results from XELOX-1 / NO16966, a randomized phase III trial in first-line metastatic. - ppt video online download

Kaplan–Meier curves of patients with stage III colon cancer in right-... | Download Scientific Diagram

PDF) PCN191 Economic Evaluation of XELOX vs FOLFOX4 as Adjuvant Treatment for Patients with Stage III Colon Cancer in south Korea | Sang-Yhun Ju - Academia.edu
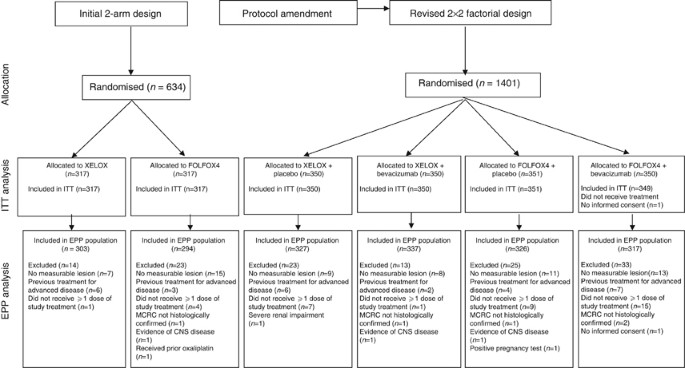
XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results | British Journal of Cancer
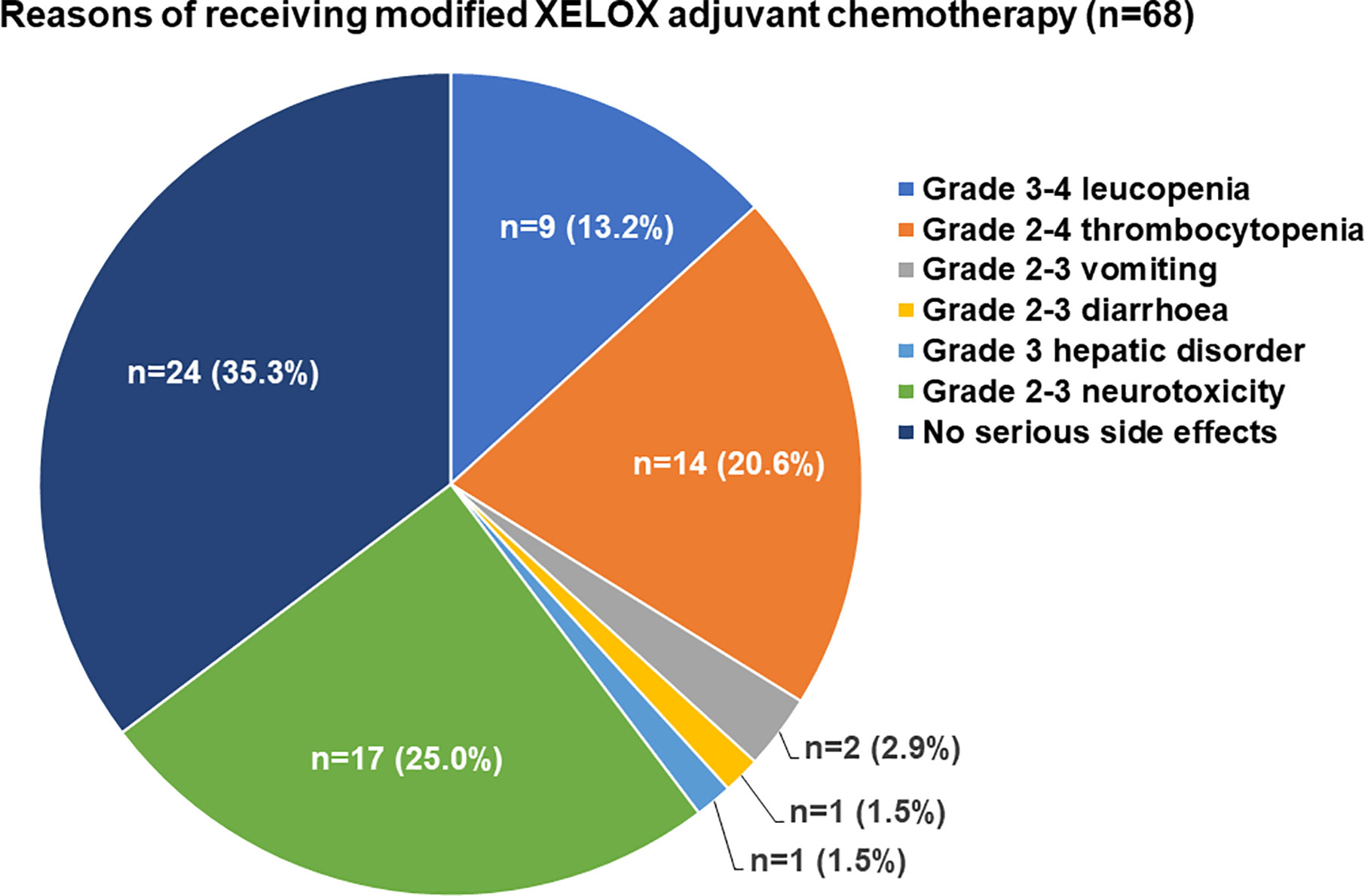
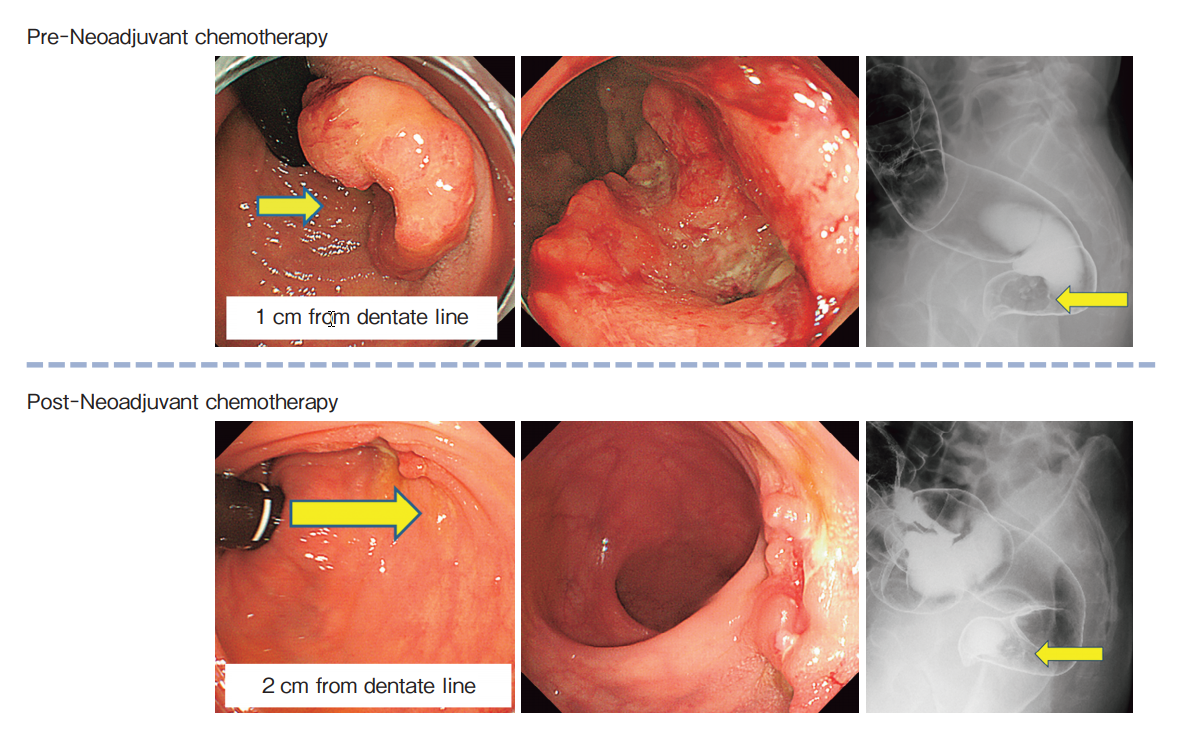
Frontiers | Feasibility Study of a Modified XELOX Adjuvant Chemotherapy for High-Recurrence Risk Patients With Operated Stage III Colon Cancer
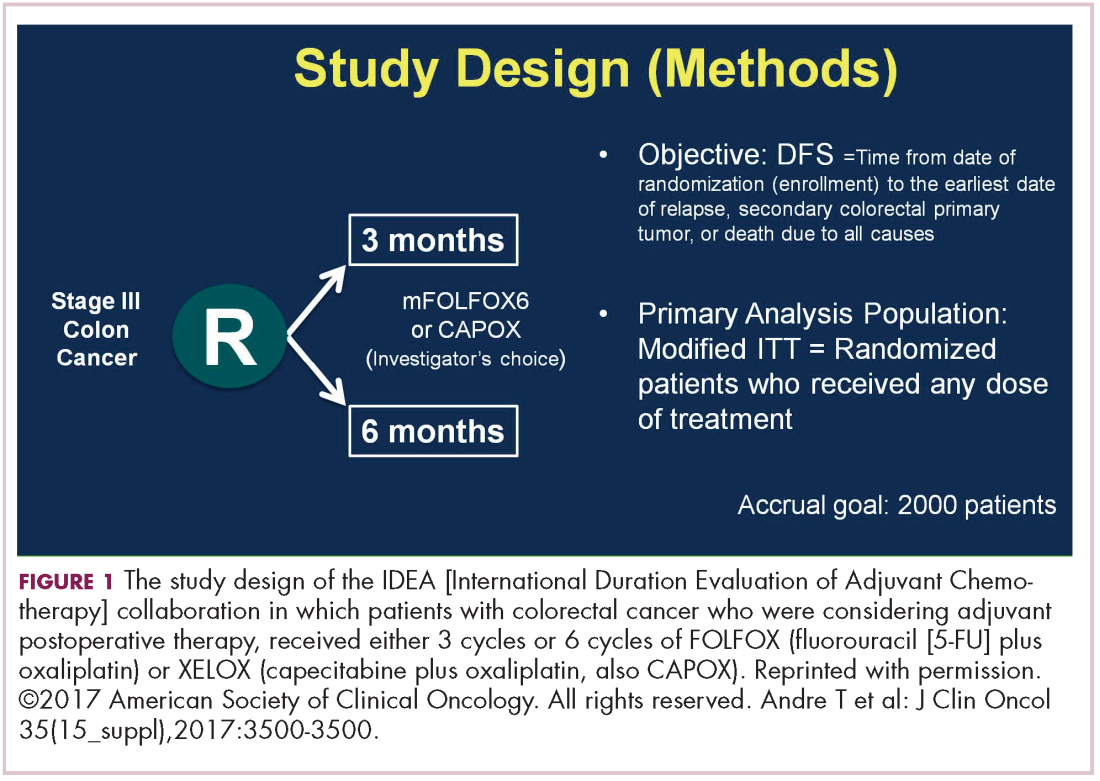
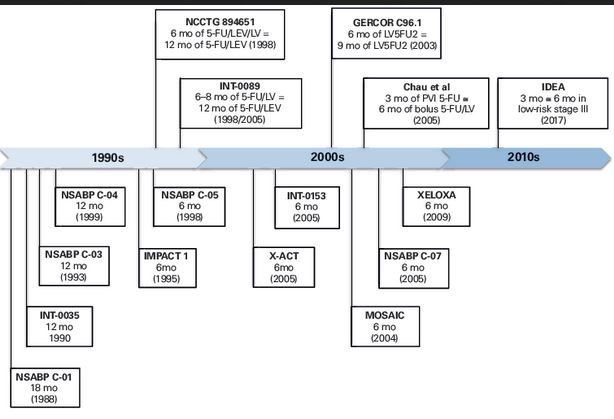
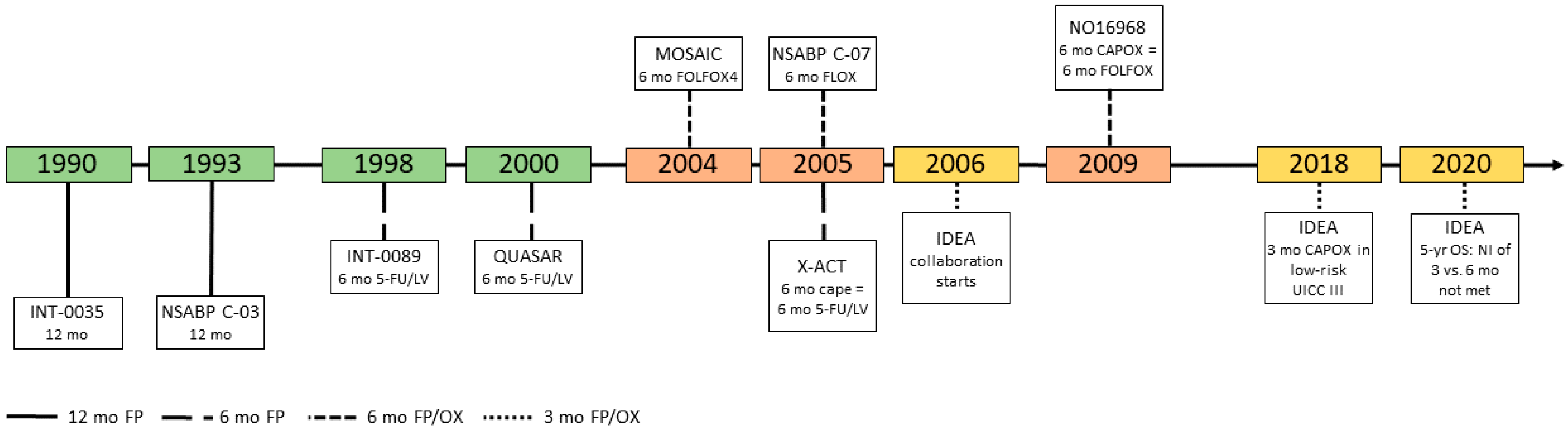
Gastrointestinal cancers: new standards of care from landmark trials | MDedge Hematology and Oncology

Safety and efficacy of a modified XELOX adjuvant regimen for patients with operated stage III colon cancer: a Chinese single-center experience | Cancer Communications | Full Text

Proposed decision algorithm for adjuvant therapy in colon cancer LN:... | Download Scientific Diagram

Table 1 from The efficacy of XELOX and FOLFOX adjuvant chemotherapy in stage III colorectal cancer patients with low preoperative serum albumin levels | Semantic Scholar

3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial - The Lancet Oncology

Figure 2 from The efficacy of XELOX and FOLFOX adjuvant chemotherapy in stage III colorectal cancer patients with low preoperative serum albumin levels | Semantic Scholar
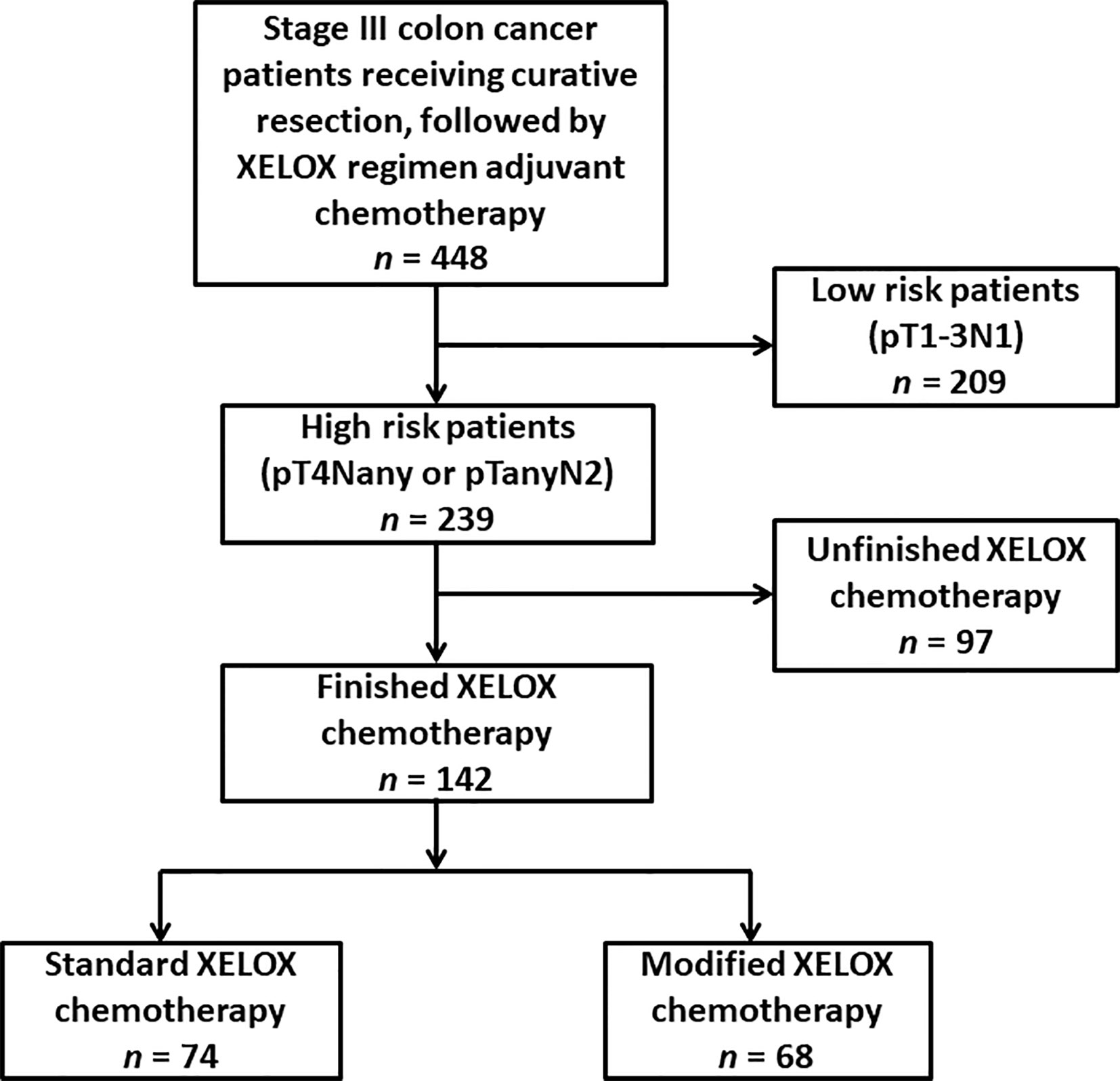
Frontiers | Feasibility Study of a Modified XELOX Adjuvant Chemotherapy for High-Recurrence Risk Patients With Operated Stage III Colon Cancer

Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. | Semantic Scholar

Median cycle of XELOX adjuvant chemotherapy received by patients for... | Download Scientific Diagram
Author: Eman Sobhy Elbanna/ Title: Adjuvant short XELOX followed by capecitabine for stage III colon cancer :